Theory of PH
The quantitative measurement of the acidity or basicity of a solution is called pH of the solution. It is defined as the negative logarithm of hydrogen ion concentration of a solution.
pH = -log [ H+ ]
If the pH of a solution is 7, the solution is called neutral. The pH of pure water is 7. Neutral solution has an equal quantity of H+ & OH− ions in pure water. The following relation exists.
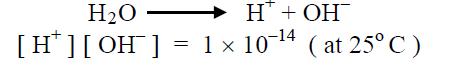
Hence pH = 7 & pOH = 7
If the pH of solution is < 7, the solution is called acidic such as HCl, H2SO4, HNO3, H3BO3, etc and if the pH > 7, the solution is basic such as NaOH, KOH, NH4OH, Ca(OH)2 etc. pH can be measured by the following methods:
(i) Colorimetric method (pH-paper/indicator)
(ii) Instrumental method (pH-meter)
In chemical laboratories, the instrumental method is most commonly used.
Interference:
High Sodium concentration above pH 10 will cause error unless special “low sodium error” electrode is used.

Apparatus:-
(i) pH meter = Model HI 8314 (HANNA)
(ii) Combined electrode = (Reference + Glass)
(iii) Beaker = 100 ml
(iv) Electrode stand
(v) Stirrer
Chemical Reagents:-
(i) Buffer solution of pH-4, pH-7 and pH-9.
(ii) Saturated KCl solution.
Reagents preparation
pH-4 buffer solution: Weigh 10.21 gram of potassium hydrogen phthalate (KHC8H4O4) and dissolve it in 1000 ml demineralised water.
pH 6.86 buffer solution: Weigh 3.4 gram potassium di-hydrogen phosphate (KH2PO4) and 3.55 gram of sodium bi phosphate (Na2HPO4 12H2O) and dissolve them in 1000 ml demineralised water.
pH 9.20 solution: Weigh 3.81 gram sodium borax (Na2B4O7.10H2O) and dissolve in 1000 ml demineralised water.
General Description
HI 8314 is a pH/mV/°C meter design for simplicity of use in taking pH, mV (ORP) and temperature measurements. The pH, mV and °C ranges are easily selected using a membrane key board on the front panel. Temperature compensation of pH is automatic when temperature probe is connected and calibration adjustments are easily made with two trimmers on the front panel. HI 8314 comes supplied with: HI 1230B combination, double junction, gel electrode, HI 7669 temperature probe, a 9 volt battery and a calibration Screwdriver.
Procedure for PH determination:-
Calibration of instrument
(i) For greatest accuracy, frequent calibration of the instrument is recommended. The instrument should be recalibrated for pH:
(a) Whenever the pH electrode or temperature probe is replaced.
(b) At least once a month.
(c) After testing aggressive chemicals.
(d) Where extreme accuracy is required.
(e) Whenever the battery has been replaced.
For accurate calibration use two beakers for each buffer solution, first one for rinsing the tip of the electrode, the second one for calibration. In this way contamination of the buffer is minimized.Switch the meter on after connecting the pH electrode and the temperature prob.Remove the protective cap from the electrode, clean the electrode with demineralised water and make it dry with tissue paper, then immerse the electrode and temperature probe into the beaker containing pH-7 buffer solution and stir gently and wait couple of minutes for thermal equilibrium.NOTE: The electrode should be submerged approximately 4cm into the solution. The temperature probe should be located close to the pH electrode.Press °C to display the temperature of the buffer.Press the pH key to read pH value. Stir gently wait for a couple of minutes.Adjust the “STD trimmer” to set the measuring indication same as the standard pH value of the solution at specific temperature (The Range read out of the meter plus the indicated value). When calibration is completed.Take out the electrode from the solution and clean it with demineralised water. Dry it with tissue paper. Now if the solution to be measured is of acidic nature i.e. pH < 7, select pH 4 standard reference solution, or if it is of alkaline nature i.e. pH > 7, select pH-9 buffer solution, put the electrode in this selected solution and adjust the “slope trimmer” to get the indication value same as the pH value of that buffer solution.
Measure again the pH 7 standard solution by using the same way as described above, Remember now not to touch the “slope” and “STD” trimmers. If the difference between the indicated value and pH value of standard solution meets the accuracy requirement for specific pH measuring, it can be concluded, that the meter is well calibrated & can be used for operation.
In case the difference does not meet the accuracy requirement, repeat the procedure of calibration again.
Measurement of Boiler sample for PH determination
In performing pH measurement, it is important to clean the electrode first. When the meter is calibrated, be sure, never to touch the “STD” and the “Slope” trimmers other wise the meter must be recalibrate. In normal situation, one calibration per day can meet the accuracy requirement.Immerse the tip of pH electrode (4cm) in the sample and stir gently for approx 30 seconds.For faster response and to avoid cross contamination of the samples, rinse the electrode tip with the solution to be tested, before taking your measurements.
Precautions
Take the pH meter reading, when the display becomes stable.Rinse the pH electrode with demineralised water, and dry it with tissue paper before putting it into a new sample.Three−fourth of the combined electrode should be filled with saturated KCl solution.Filling hole of the electrode should be kept open during measuring of pH values.It is better to have the temperature of the sample to be measured same as the temperature of the standard solution used for calibration. This will help to minimize the measuring error by the electrode and increase accuracy.
References Instruction Manual of pH model HI 8314(HANNA).
 Boilersinfo Boiler and Mechanical Power Digital Library
Boilersinfo Boiler and Mechanical Power Digital Library