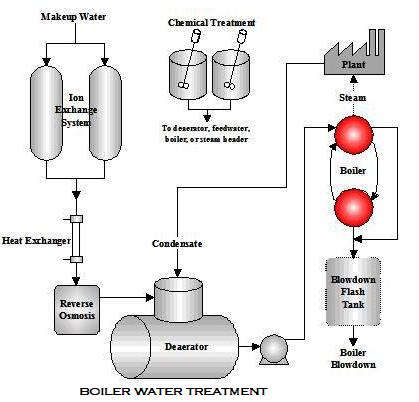
Boiler Water Treatment is necessary to Produce quality steam on demand and depends on properly managed water treatment to control steam purity, deposits, and corrosion. There are two main types of boiler feed water treatment Internal boiler Water Treatment method and external boiler water treatment. A boiler is the sump of the boiler system. It ultimately receives all of the pre-boiler contaminants. Boiler performance, efficiency, and service life are direct products of selecting and controlling feedwater used in the boiler.
When BFW boiler feedwater enters the boiler, the elevated temperatures and pressures cause the components of water to behave differently. Most of the components in the feedwater are soluble. However, under heat and pressure, most of the soluble components come out of the solution as particulate solids, sometimes in crystallized forms and other times as amorphous particles. When the solubility of a specific component in water is exceeded, scale or deposits develop. The boiler water must be sufficiently free of deposit-forming solids to allow rapid and efficient heat transfer and it must not be corrosive to the boiler metal.
Deposit Control in Boiler Water Treatment
Deposits in boilers may result from hardness contamination of feed water and corrosion products from the condensate and feedwater system. Hardness contamination of the feed water may arise due to a deficient softener system. Deposits and corrosion result in efficiency losses and may result in boiler tube failures and inability to produce steam. Deposits act as insulators and slow heat transfer. Large amounts of deposits throughout the boiler could reduce the heat transfer enough to reduce the boiler efficiency significantly. Different type of deposits affects boiler efficiency differently. Thus it may be useful to analyze the deposits for their characteristics. The insulating effect of deposits causes the boiler metal temperature to rise and may lead to tube failure by overheating.
Impurities causing deposits
The most important chemicals contained in water that influence the formation of deposits in the boilers are the salts of calcium and magnesium, which are known as hardness salts. Calcium and magnesium bicarbonate dissolve in water to form an alkaline solution and these salts are known as alkaline hardness. They decompose upon heating, releasing carbon dioxide and forming a soft sludge, which settles out These are called temporary hardness that can be removed by boiling. Calcium and magnesium sulfates, chlorides and nitrates, etc. when dissolved in water are chemically neutral and are known as non-alkaline hardness. These are called permanent hardness and form bard scales on boiler surfaces, which are difficult to remove. Non-alkalinity hardness chemicals fall out of the solution due to a reduction in solubility as the temperature rises, by concentration due to evaporation which takes place within the boiler, or by the chemical change to a less soluble compound.
Silica
The presence of silica in boiler water can rise to the formation of bard silicate scales. It can also associate with calcium and magnesium salts, forming calcium and magnesium silicates of very low thermal conductivity. Silica can give rise to deposits on steam turbine blades which can reduce plant efficiency after being carried over either in droplets of water in steam or in the volatile form in steam at higher pressures. Two major types of boiler water treatment are Internal water treatment and External water treatment.
Internal Water Treatment
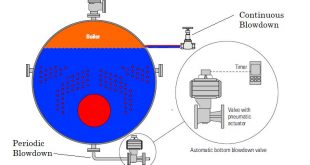
Boiler Internal treatment is carried out by adding chemicals to the boiler to prevent the formation of scale by converting the scale-forming compounds to free-flowing sludge, which can be removed by taking blowdown. This method is limited to boilers small package low-pressure boilers, where feed water is low in hardness salts, to low pressures- high TDS content in boiler water is tolerated, and when an only a small quantity of water is required to be treated. If these conditions are not applied, then high rates of blowdown are required to dispose off the sludge. They become uneconomical from heat and water loss consideration. both internal and external water treatment are recommended. Only inter boiler water treatment method is not enough to get good results.
External Water Treatment
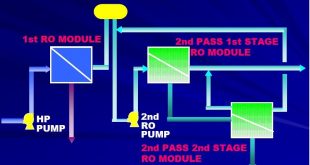
External treatment is used to remove suspended solids, dissolved solids (particularly the calcium and magnesium ions which are major causes of scale formation), and dissolved gases (oxygen and carbon dioxide). The external treatment processes available are the ion exchange or demineralization process, RO reverse osmosis, and Deaeration. Before any of these are used, it is necessary to remove suspended solids and turbidity from the raw water, because these may foul the resins used in the subsequent treatment sections.
Methods of pre-treatment sedimentation, clarification, coagulation, and aeration of water will be discussed later.
 Boilersinfo Boiler and Mechanical Power Digital Library
Boilersinfo Boiler and Mechanical Power Digital Library